CCG 203769
CAS No. 410074-60-1
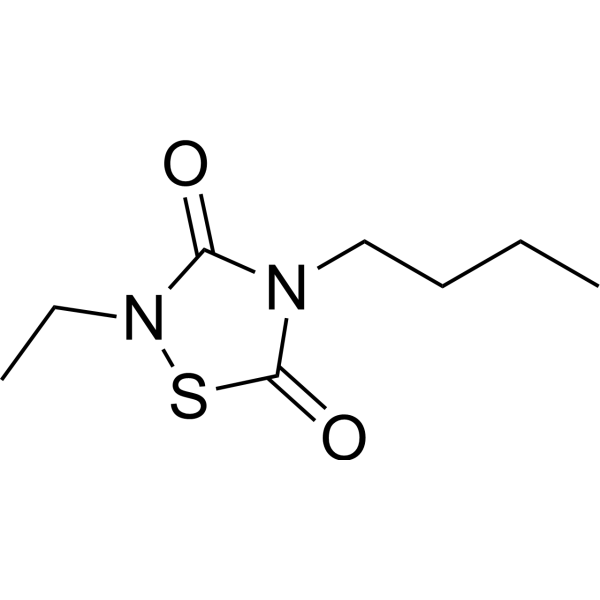
CCG 203769( Thiadiazolidinone (TDZD) deriv. 6 | RGS4 inhibitor 11b | 4-butyl-2-ethyl-1,2,4-thiadiazolidine-3,5-dione )
Catalog No. M26093 CAS No. 410074-60-1
CCG 203769 is a selective inhibitor of RGS4 with an IC50 of 17 nM for the RGS4-Gαo protein-protein interaction.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 2MG | 152 | Get Quote |


|
| 5MG | 260 | Get Quote |


|
| 10MG | 447 | Get Quote |


|
| 25MG | 714 | Get Quote |


|
| 50MG | 1017 | Get Quote |


|
| 100MG | 1368 | Get Quote |


|
| 200MG | Get Quote | Get Quote |


|
| 500MG | Get Quote | Get Quote |


|
| 1G | Get Quote | Get Quote |


|
Biological Information
-
Product NameCCG 203769
-
NoteResearch use only, not for human use.
-
Brief DescriptionCCG 203769 is a selective inhibitor of RGS4 with an IC50 of 17 nM for the RGS4-Gαo protein-protein interaction.
-
DescriptionCCG 203769 is a selective inhibitor of RGS4 with an IC50 of 17 nM for the RGS4-Gαo protein-protein interaction.(In Vitro):CCG 203769 displays dramatic selectivity (8- to >5000-fold) for RGS4 over other RGS proteins with IC50s of 140 nM, 6 μM, and 79 μM for RGS19, RGS16, and RGS8. CCG 203769 inhibits GSK-3β with an IC50 of 5 μM. CCG 203769 enhances Gαq-dependent cellular Ca2+ signaling in an RGS4-dependent manner and inhibits RGS/Gαo binding in an RGS-selective manner. CCG 203769 also blocks the GTPase accelerating protein (GAP) activity of RGS4. CCG 203769 inhibits the effect of GTP hydrolysis stimulated by RGS4 with an IC50<1 μM in single-turnover and steady-state GTPase experiments.(In Vivo):CCG 203769 (10 mg/kg, i.v.), administered immediately prior to Carbamoylcholine chloride(0.1 mg/kg, i.p.), significantly potentiates the bradycardic effect. CCG 203769 (1-10 mg/kg) reverses the increased hang time caused by raclopride administration in rats. CCG 203769 (0.1-10 mg/kg) reverses the raclopride-induced paw drag in mice.
-
In VitroCCG 203769 also displays dramatic selectivity (8- to >5000-fold) for RGS4 over other RGS proteins.CCG 203769 inhibits RGS19 with an IC50 of 140 nM (8-fold selective for RGS4) and 6 μM for RGS16 (350-fold selective for RGS4). The closely related RGS8 is very weakly inhibited (IC50>60 μM) providing >4500-fold selectivity for RGS4. CCG 203769 inhibits GSK-3β with an IC50 value of 5 μM. CCG 203769 does not inhibit the cysteine protease papain at 100 μM. CCG 203769 does not inhibit RGS7, which lacks cysteines in the RGS domain. CCG 203769 inhibits RGS/Gαo binding in an RGS-selective manner. CCG 203769 enhances Gαq-dependent cellular Ca2+ signaling in an RGS4-dependent manner. CCG 203769 also blocks the GTPase accelerating protein (GAP) activity of RGS4. In single-turnover and steady-state GTPase experiments with Gαo and Gαi1, the rate of GTP hydrolysis is strongly stimulated by RGS4, and this effect is inhibited by CCG 203769 with an IC50<1 μM.
-
In VivoTo determine whether this genetic disruption of RGS4 function can be replicated pharmacologically, CCG 203769 is tested for effects on Carbamoylcholine chloride-mediated bradycardia in conscious, unrestrained rats. Carbamoylcholine chloride (0.1 mg/kg, IP) produces a modest decrease in heart rate compared to that of a saline vehicle control. CCG 203769 (10 mg/kg, IV) has no significant effect upon heart rate when given alone. However, CCG 203769, administered immediately prior to Carbamoylcholine chloride, significantly potentiates the bradycardic effect (p < 0.05). Given the functional role of RGS4 in Parkinson’s disease models, CCG 203769 is tested in a pharmacologic model of D2 antagonist-induced bradykinesia. Raclopride administration in rats causes increased hang time in the bar test, which is rapidly reversed by doses of CCG 203769 ranging from 0.1 to 10 mg/kg. The lowest dose, 0.01 mg/kg has no effect, while 0.1 mg/kg produces a submaximal effect. The higher doses, 1 and 10 mg/kg, produce equivalent effects. Similarly, the raclopride-induced paw drag in mice is reversed by 0.1-10 mg/kg CCG 203769.
-
SynonymsThiadiazolidinone (TDZD) deriv. 6 | RGS4 inhibitor 11b | 4-butyl-2-ethyl-1,2,4-thiadiazolidine-3,5-dione
-
PathwayPI3K/Akt/mTOR signaling
-
TargetGSK-3
-
Recptor5-HT1| 5-HT2A| 5-HT2C| D2| D4| α1 receptor
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number410074-60-1
-
Formula Weight202.27
-
Molecular FormulaC8H14N2O2S
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 62.5 mg/mL (308.99 mM)
-
SMILESO=C1SN(C(=O)N1CCCC)CC
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference



-
GSK-3β inhibitor 14
GSK-3β inhibitor 14 (1,5-Benzothiazepin-4(5H)-one, 2,3-dihydro-2-methyl-5-(phenylmethyl)-) is a weak GSK-3β inhibitor, IC50﹥ 100μM.
-
ABC 1183
ABC1183 is a potent, selective, orally active GSK3α/β and CDK9 inhibitor with IC50 of 327/657 nM and 321 nM (CDK9/cyclin T1).
-
9-ING-41
9-ING-41 is a glycogen synthase kinase-3 inhibitor.9-ING-41 (2, 4 μM; 48 hours) decreases neuroblastoma cell viability induces apoptosis[2]. 9-ING-41 (0.1-1 μM) inhibits GSK-3 leading to a decreased expression of the NF-κB target XIAP and significant apoptosis in neuroblastoma cells as shown by PARP cleavage, an apoptosis marker[1]. 9-ING-41 (0.5, 1.0, 1.5, 2.0 μM) inhibits the proliferation rate of all TCL and MCL lines with concentrations as low as 1.0 mM.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


